
Submit Projects

Any member of the faculty, staff, or student body who proposes to engage in research activity involving human subjects at UTEP must submit their project for review by the Institutional Review Board.
External researchers interested in research involving UTEP's students, faculty, or staff must request permission from UTEP's IRB.
IRBNet Registration
START
Go to irbnet.org
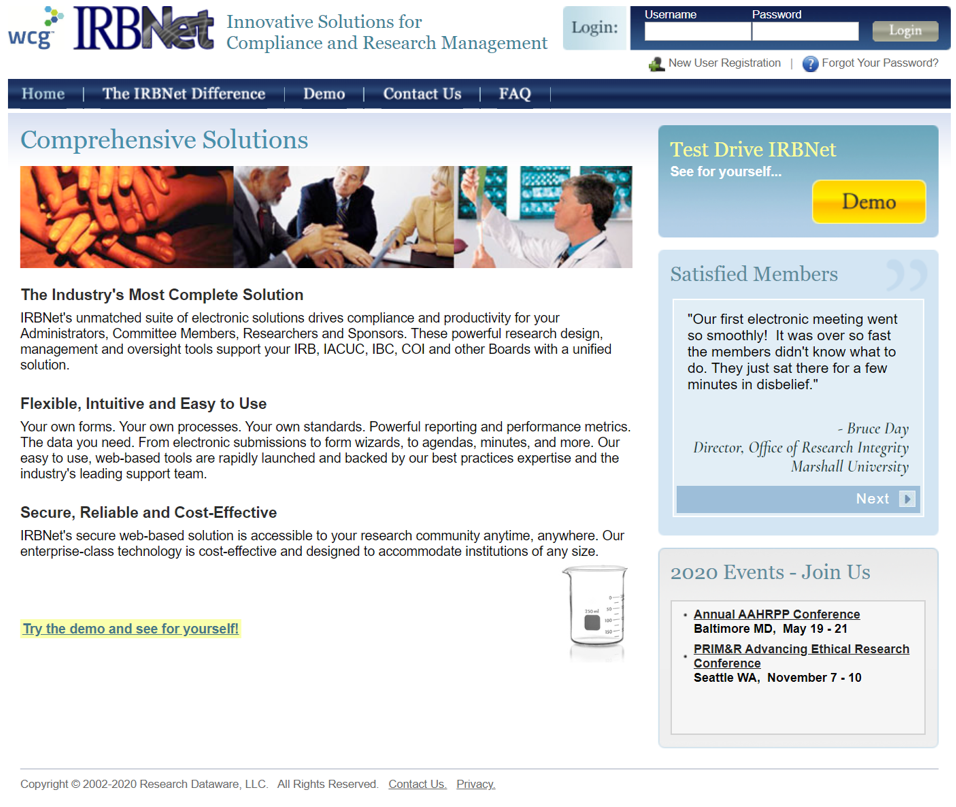
Click New User Registration
1
Start Registration
It is recommended that your first and last name is consistent with your name at UTEP & CITI.
2
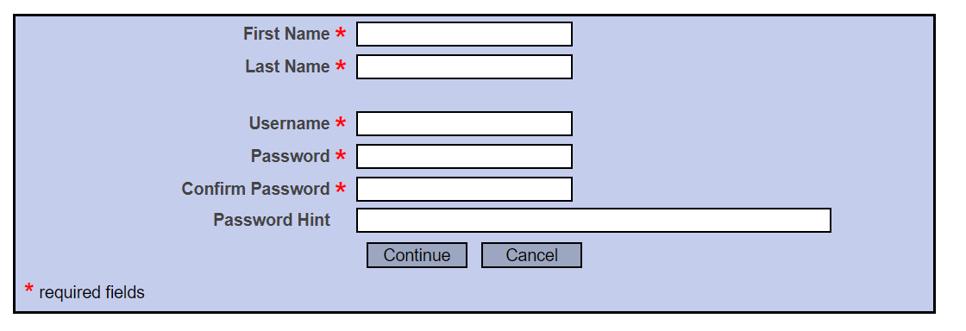
To Affiliate With UTEP:
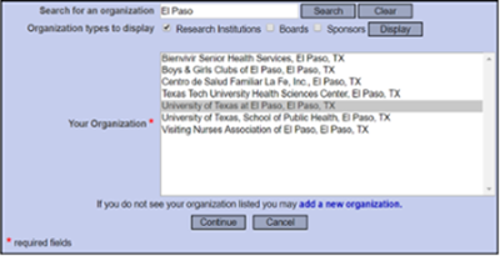
Search "El Paso" and select University of Texas at El Paso, El Paso, TX
3
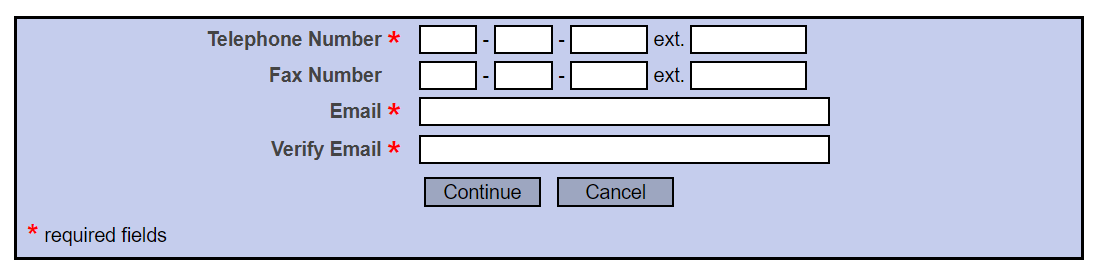
Use your most available contact number and e-mail address. It is recommended to use UTEP credentials as all IRB correspondence will be sent to this e-mail.
4
Complete registration and verify to activate your account via email.
Submit Your Project
New Project
Common Documents Submitted
Research Protocol Application
Informed Consent Form
Relevant study instruments (questionnaires, surveys, interview questions, etc)
Advertisements (Recruiting Communications)
CITI Training
As Needed Documents Submitted
Study Information Sheet
Informed Consent Prospective Deception
Parental Permission Form
Child Assent Form
Site Approval Letters
Letter of Collaboration
Reliance Agreements
Funding/
Grant
Common Documents Submitted
Request for 45 CFR 46.118
CITI Training
One complete copy of the grant application, and the identification number assigned to it by ORSP
Funding Source supporting documents
Amendment/
Modification
Common Documents Submitted
Amendment Request Form
IRB Project Team Form (Addition/Deletion)
CITI Training
Any previously submitted documents affected by amendment/modification
Project Status
(Continuing Review/Progress Report)Common Documents Submitted
Project Status Report
Research Protocol Application
Informed Consent Form
CITI Training
Protocol
Deviation/
Violation
Common Documents Submitted
Project Status Report
CITI Training
Adverse Event
Common Documents Submitted
Project Status Report
CITI Training
Unanticipated
Problem
Common Documents Submitted
Project Status Report
CITI Training
Closure/Final Report
Common Documents Submitted
Closure Termination Form
1
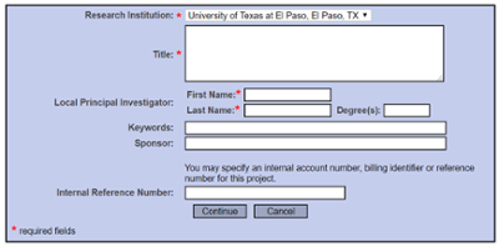
Create a new project
Insert the title of your project. Please ensure the title is consistent with the protocol application, consent documents, etc.
2
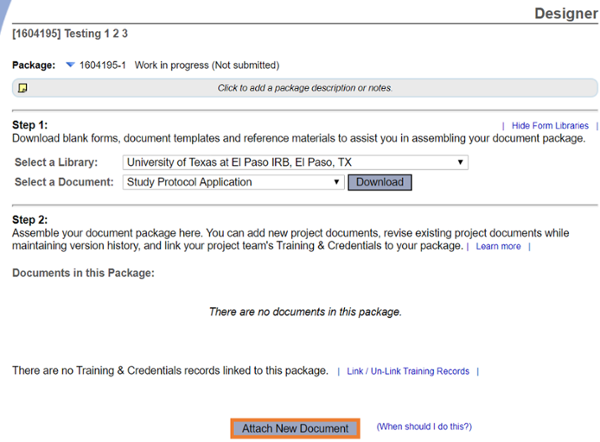
Go to Designer
Attach all study documents. Please use the current versions available for download in Designer.
3
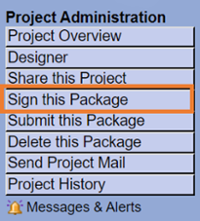
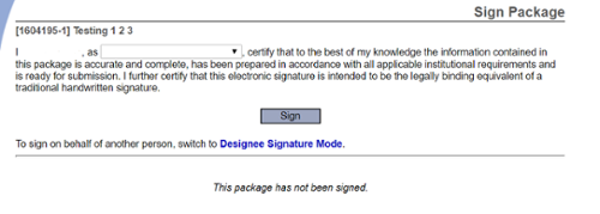
Sign this Package
Sign the package, be sure to choose the role that best describes your role in the research project, e.g. Principal Investigator, Research Coordinator, etc.
4
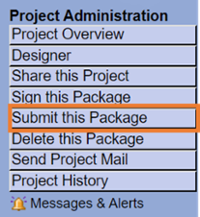
Submit this Package
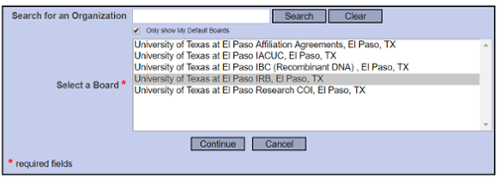
Select “University of Texas at El Paso IRB, El Paso, TX” and continue to submit.
 This next step is mandatory for students.
This next step is mandatory for students.
Optional, but recommended, for Faculty/Staff.
EXTRA
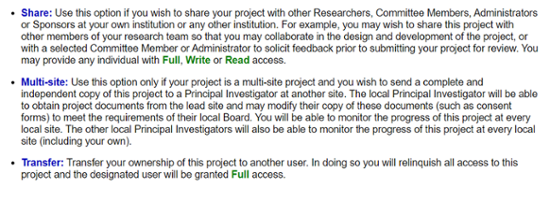
Share this Project.
Select Share.
Students must share their project with their sponsor/faculty advisor.
EXTRA
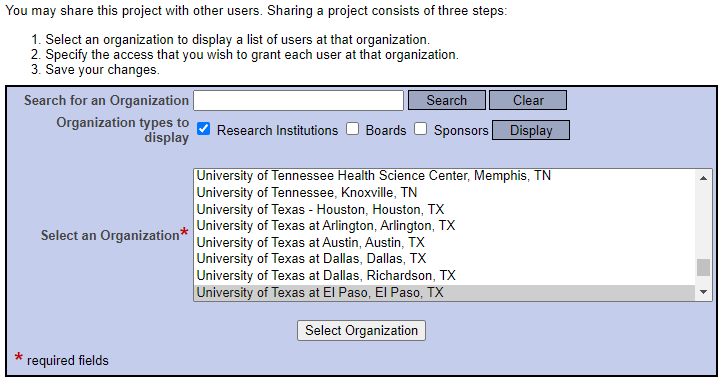
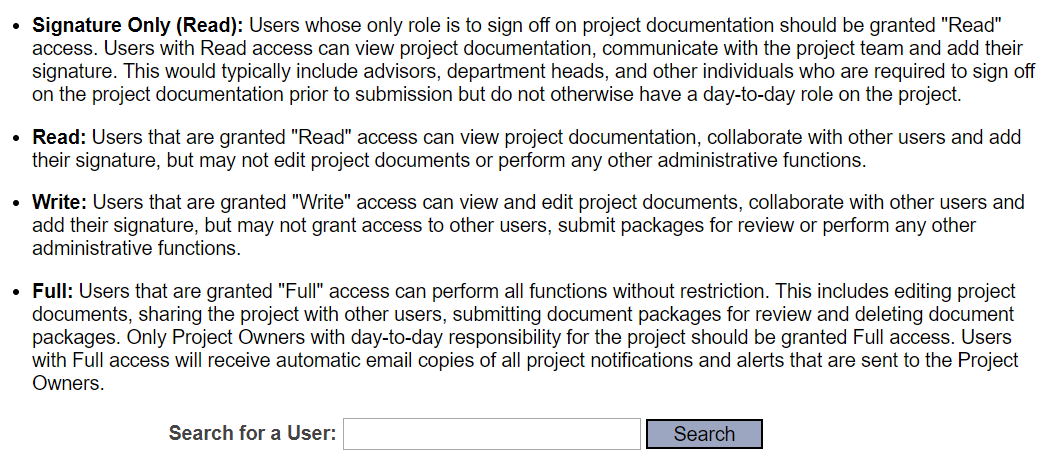
Share this Project.
Select “University of Texas at El Paso, El Paso, TX” as your organization.
Search for your faculty advisor by their last name.
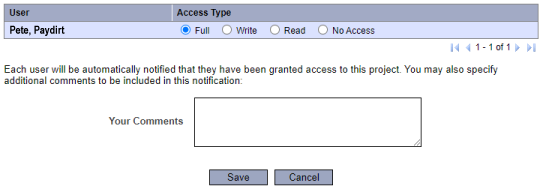
EXTRA
Share this Project.
Email Faculty Advisor.
Send a gentle reminder to your sponsor/faculty advisor to review and electronically sign your IRB project submission.
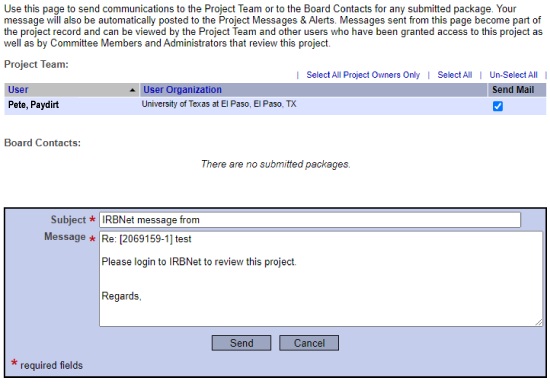

The principal investigator must sign and submit the project before it is eligible for review.
We suggest you send the submission information to your Department Chair as a courtesy.
Student PIs must share their projects with the sponsor/faculty advisor. Sponsor/Faculty Advisor must sign before the project is eligible for review.
Additional Guidance
Definition(s):
- Deception: involves deliberately misleading subjects about research purposes or activities by intentionally providing inaccurate or false information and/or by withholding information, with the intention of misleading subjects about the research purpose or procedures.
- Behavioral intervention: involves the performance of a cognitive, intellectual, educational, or behavioral task; or the manipulation of the subject’s physical, sensory, social, or emotional environment.
Background:
- Any research in which information is withheld until subjects have participated to some degree should be considered as a deception study.
- This type of research methodology is sometimes used to:
- Improve study validity,
- Assure study integrity, or
- Allow data collection that would otherwise be unobtainable because of defensiveness, shame, etc.
Guidelines:
-
Regarding the design, review and conduct of studies involving deception:
- Use of deception is usually only acceptable for studies that are minimal risk.
- The use of deception should have no adverse effects on the well-being of subjects.
- The IRB has sufficient information to determine that the value of the research outweighs the risk of waiving some aspects of the requirement for full disclosure in the informed consent process.
- There is no reasonable alternative to scientifically and effectively address the research question without the use of deception.
- Subjects are not deceived about any aspect of the study that would alter their willingness to participate.
- As soon as it is appropriate, debriefing should be accomplished and the deception explained to subjects.
- When appropriate, subjects should be informed prospectively of the use of deception and consent to its use.
- During debriefing inform subjects of their right to withdraw their data, if they wish, and how that will be accomplished.
P.I. Requirements:
- Explain the reason(s) for use of deception in the study design. Specifically, address why complete disclosure would compromise the scientific validity of the study.
- Describe the extent of the deception in detail and how it relates to the study aims and design.
- Justify and discuss how the proposed research, involving deception involves no more than minimal risk to subjects. Consider all levels of increased risk subjects could experience as a result of the deception.
- Justify and discuss why there are no feasible or scientifically valid alternative methods, which do not involve deception, to conduct the research.
- Describe the methods for prompt disclosure to debrief subjects. This should be accomplished as soon as possible after subjects complete research related activities.
- Describe how you will assure that subjects leave with a clear and accurate understanding of the deception and the reasons for using this methodology. If debriefing is not planned, justify why.
Potential Risks:
-
There are several potential risks associated with use of deception and these should be considered when designing the study:
- Subjects may feel that they were coerced to act against their will. If so and if they had been completely informed, they may have chosen not to participate.
- Subjects may feel ashamed, guilty, stressed, or embarrassed because they now have knowledge about themselves that they otherwise would not have known or would not want to know.
- Subjects may feel a loss of control that will cause distrust and suspicion regarding human subjects research in general.
- The research may undermine the trust in professional standards governing human subjects research.
The IRB must review and approve all advertisements that will be used to recruit subjects to a specific research study.
Generally, advertisements used to recruit research subjects should be limited to information that a potential subject would need to determine if they are eligible and interested in participating.
The ads should include information such as:
- the name and address of the research facility;
- the condition or disease that will be the focus of the research;
- the purpose of the research with reference to the fact that the study is investigational;
- a summary of criteria for eligibility to participate;
- the time and other commitments that will be required of the subject; and
- the location of the study and the office to contact for further information.
Ads may state that reimbursement for time, travel, etc. will be given.
The ads should not:
- contain explicit or implicit claims of safety and efficacy or equivalency or superiority to other approved treatments
- emphasize the amount of reimbursement that subjects will receive.
- promise a favorable outcome or benefits
When the advertisements conform to the above guidelines, they may be approved for any advertising format, e.g., posted flyers, newspaper advertisements, Internet advertisements, radio/television, slides shown prior to films at movie theaters.
To avoid multiple requests for review and approval, investigators should specify all anticipated advertising formats in their original request. A template is available on our Forms page.
Mandatory Reporting
Policy:
Texas Education Code, Chapter 51, Subchapters E-2 and E-3, is a Texas State Law that requires all employees (both faculty and staff) at a public or private post-secondary institution to promptly report to the Title IX Coordinator any knowledge of any incidents of sexual assault, sexual harassment, dating violence, or stalking "committed by or against a person who was a student enrolled at or an employee of the institution at the time of the incident".
Do I have a responsibility to report an incident I learn about during the conduct of a research project?
Yes. However, employees of UTEP conducting an IRB approved research project, are considered confidential employees for the purposes of the research project.If you find out about a reportable incident (e.g., sexual assault, sexual harassment, dating violence, and/or stalking), you will only have to report census-level data:
- date reported
- type of incident
- location of incident (if known)
- date/time of incident (if known)
- faculty/staff member reporting
You are not required to report any individually identifiable information about the incident or the individual making the disclosure.
Does being designated as a confidential employee for SB 212 change my status as a mandated reporter?
No. You are still required to report incidents you witness or receive information about to the Title IX Coordinator. Your confidential employee designation only applies to information received through the course and scope of the research project.
Should I say anything about reporting requirements for SB 212 in my informed consent process?
Yes. Include the following statement in your informed consent document if, in your opinion, it is likely the research may reveal reportable incidents (e.g., sexual assault, sexual harassment, dating violence, and/or stalking):
Texas Education Code, Chapter 51, Subchapters E-2 and E-3, requires reporting incidents of sexual assault, sexual harassment, dating violence, or stalking committed by or against a person who was a student enrolled at or an employee of UTEP at the time of the incident. However, the researchers working on this study have been designated as confidential employees. This means that if we learn about any incidents of sexual assault, sexual harassment, dating violence, or stalking, we are only required to report the type of incident reported and the date we learn about the incident. We will not report any information that could identify you.
Policy:
Texas law requires the reporting of suspected child abuse or neglect. If there is a reasonable chance that information may be elicited concerning child or elder abuse or neglect, or potentially dangerous future behavior to others as part of the research protocol, the following disclosure must be made:
Under certain situations, we may break confidentiality. Texas law requires that the disclosure of child or elder abuse, neglect, or potentially dangerous future behavior to yourself or others, be reported to the proper authorities. If during the study we learn about this information, we will report it to the appropriate authorities including the police and/or the Texas Department of Family and Protective Services, and/or an emergency medical facility.
Policy:
Any payments (cash or gift card) made by UTEP personnel must be reported to VPBA.
The following disclosure must be made on consent forms:
For reporting purposes, you are required to provide a Social Security Number (SSN) or Tax Information Number (TIN) to the Primary Investigator (PI) to receive cash or gift cards from UTEP. This information is not a part of your research data and will be shared only with the applicable UTEP office.
Policy:
Use these questions to determine the difference between a clinical study and a clinical trial:
- Does the study involve human participants?
- Are the participants prospectively assigned to an intervention?
- Is the study designed to evaluate the effect of the intervention on the participants?
- Is the effect being evaluated a health-related biomedical or behavioral outcome?
If yes, your study meets the NIH definition of a clinical trial, even if…
- You are studying healthy participants
- Your study does not have a comparison group (e.g., placebo or control)
- Your study is only designed to assess the pharmacokinetics, safety, and/or maximum tolerated dose of an investigational drug
- Your study is utilizing a behavioral intervention
- Only one aim or sub-aim of your study meets the clinical trial definition
Studies intended solely to refine measures are not considered clinical trials.
Studies that involve secondary research with biological specimens or health information are not clinical trials.
All applicable clinical trials must be registered on clinicaltrials.gov. The following language is required for clinical trials:
A description of this study will be available on http://www.ClinicalTrials.gov as required by U.S. law. This web site will not include information that can identify you. At most, the web site will include a summary of the results. You can search this web site at any time.
Types of IRB Review
Review Time
10 - 14 days*
*Timeframe varies depending on the completeness or complexity of the project.Only the IRB Chair and/or designee need to review the project. The project must fall into 1 of the 8 categories as defined by 45 CFR 46 and not be higher than minimal risk. Exempt studies are valid for a two-year period. Changes to the project must be submitted to the IRB prior to implementation.
