
IRB Training Requirements

All UTEP researchers (faculty, staff, and students) and outside collaborators who will be conducting human subjects’ research must complete human subject research ethics training in order to conduct research with human participants.
Training can be completed through the Collaborative Institutional Training Initiative (CITI). Once completed, the training is valid for three years and accepted at most universities across the United States.
As you register and create an account, you will be asked supplemental questions about your role with human subjects and research
-
Nursing, Pharmacy & Allied Health Researchers
-
Social & Behavioral Researchers
-
IRB Members- ONLY for IRB Committee Members
START
Go to citiprogram.org
Click Register
1
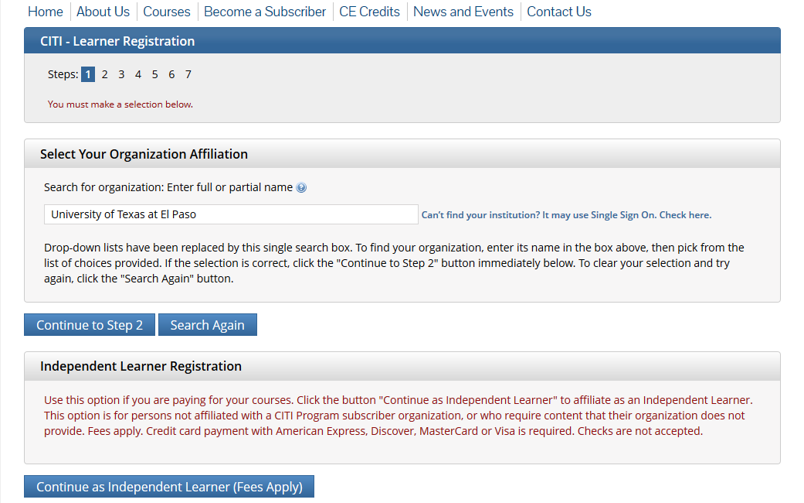
Start Registration
Affiliate with UTEP
2
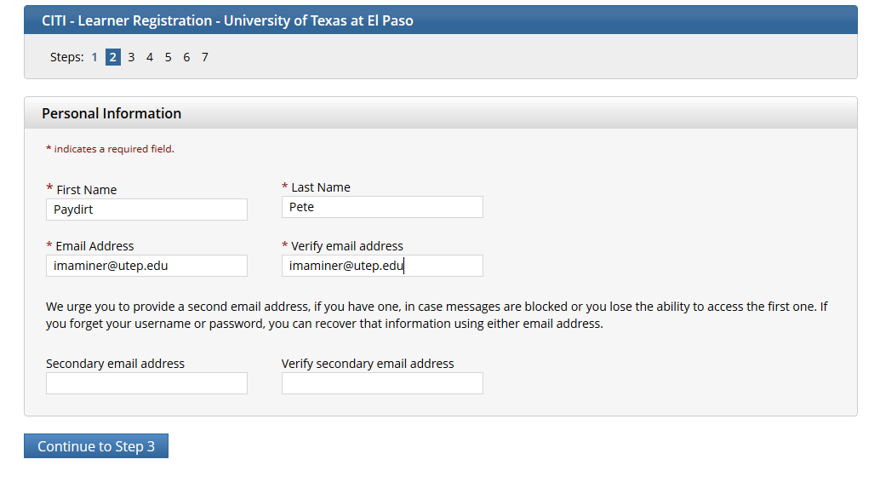
Fill out personal information
3
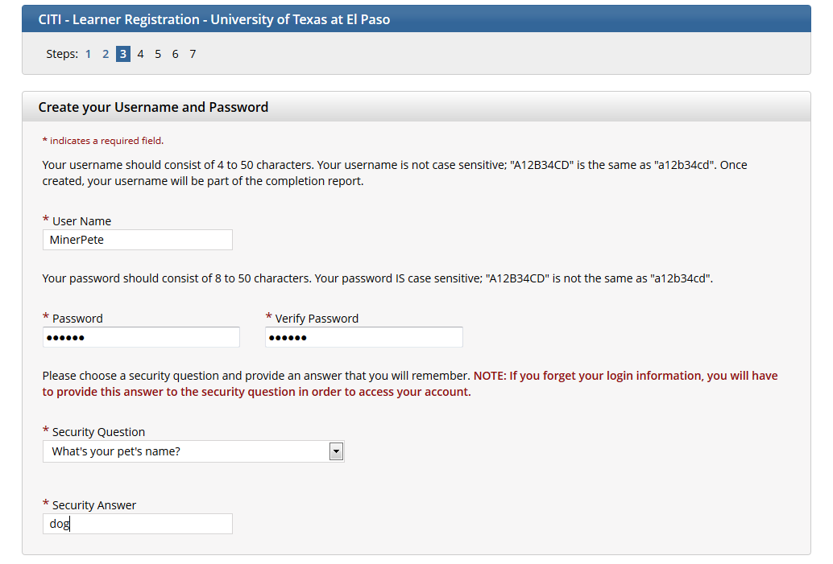
Create your username and password
4
Fill out country of residence
5
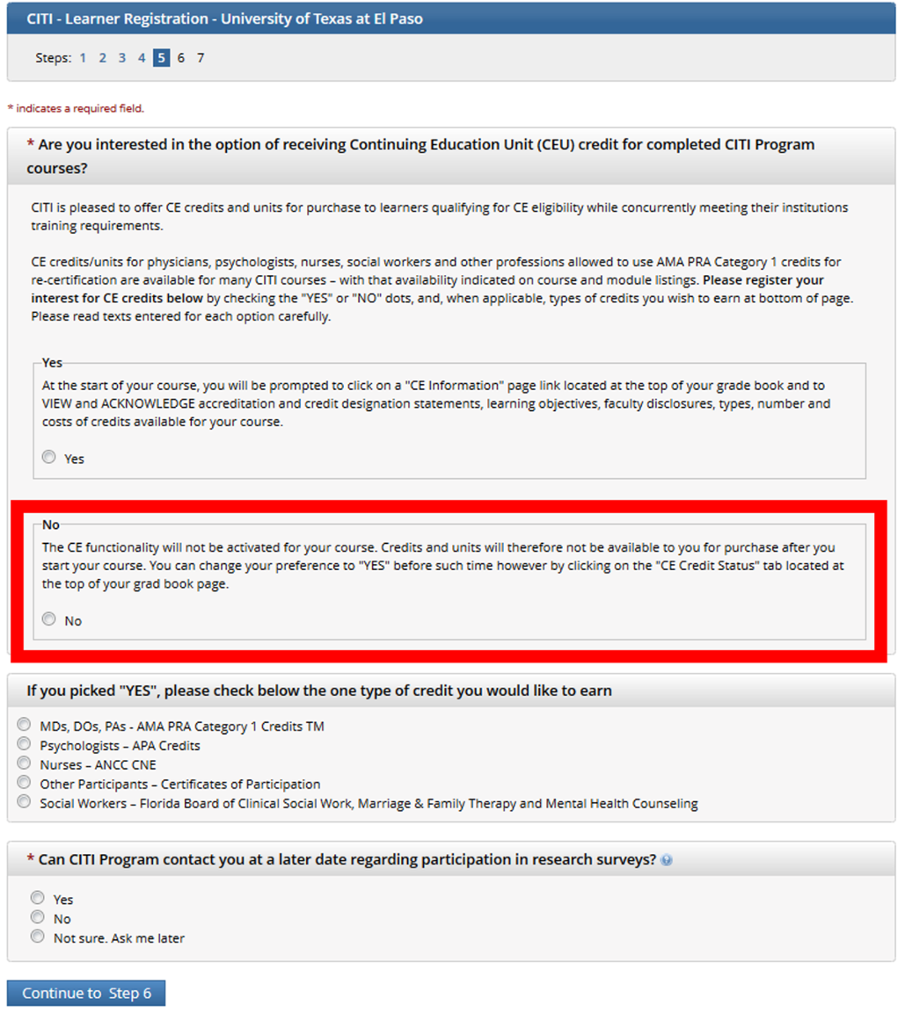
Make sure you say NO to CEUs
6
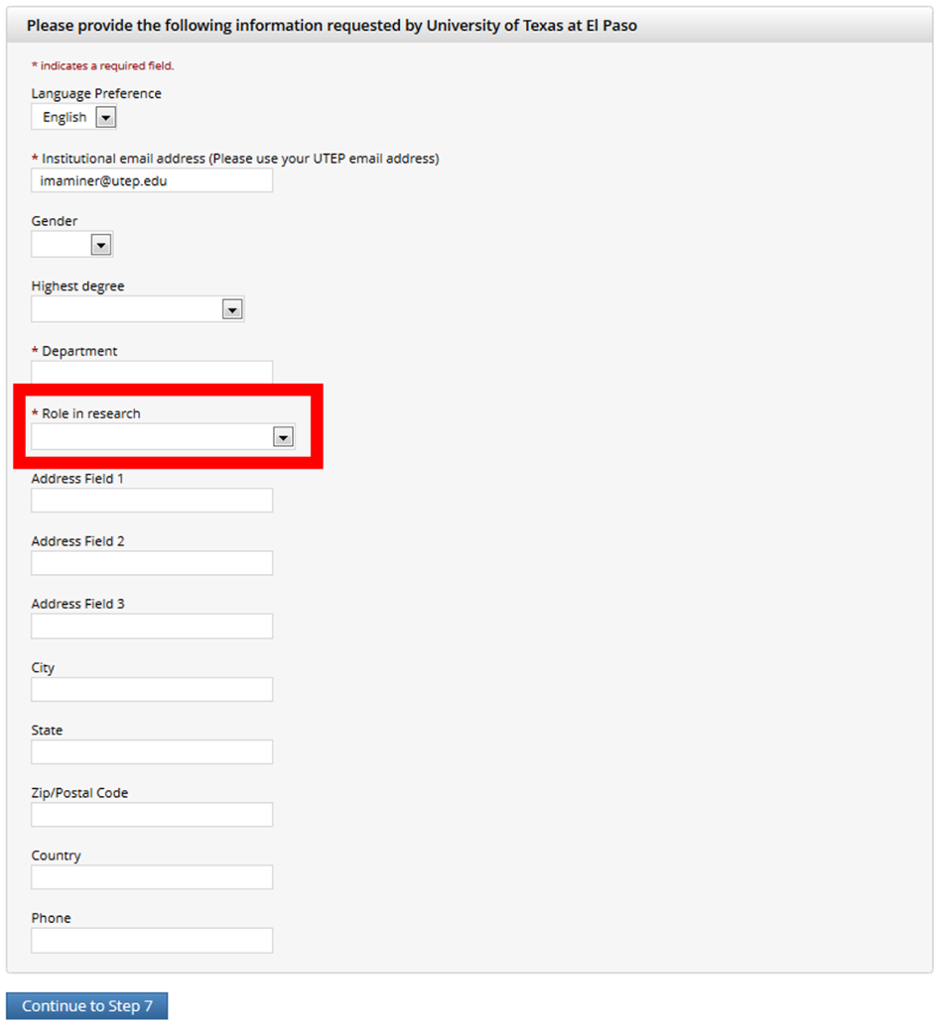
Select your role in research
Use UTEP’s Address:
500 West University Ave.
El Paso, Texas 79968
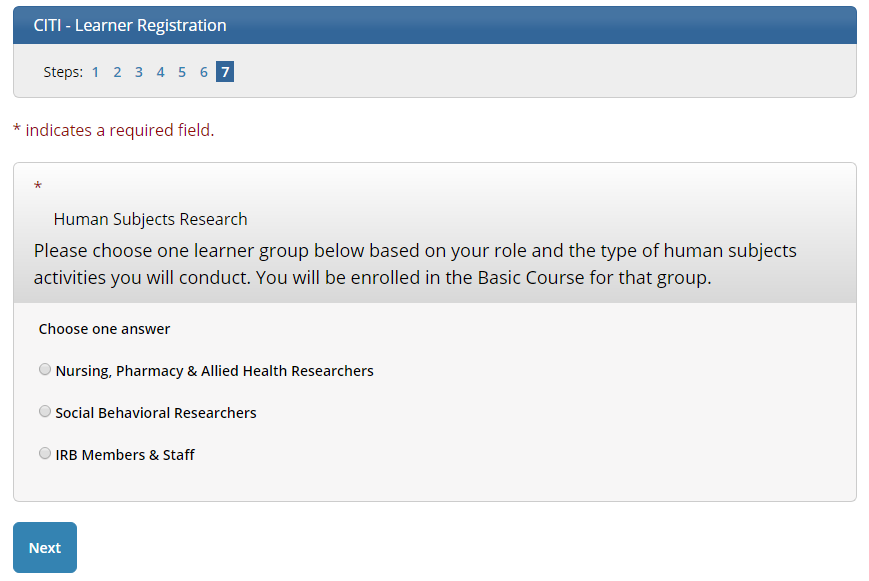
7
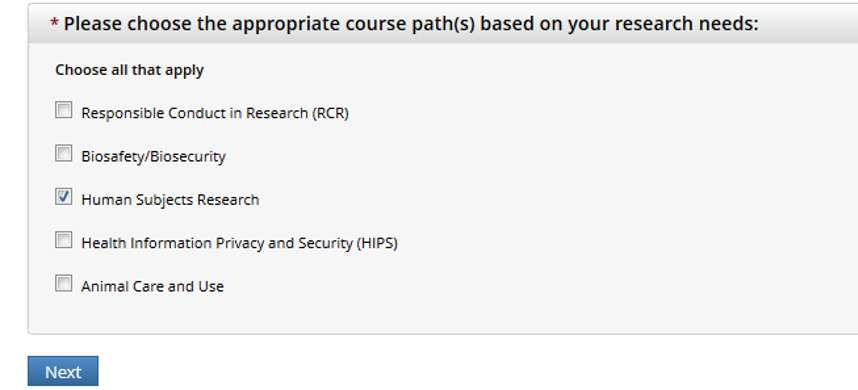
Choose Human Subject Research
Most people should select Social Behavioral Researchers
END
Finalize Registration
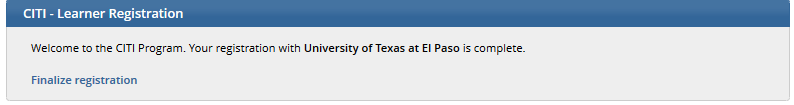
If you have completed IRB ethics training in the past three years, please follow the instructions below to upload your IRB training certificate into IRBNet.
- Log into IRBNet.
- At the top of the screen, click on “User Profile”.
- Scroll down to “Training & Credentials” and click on “Add a New Training & Credentials Record”.
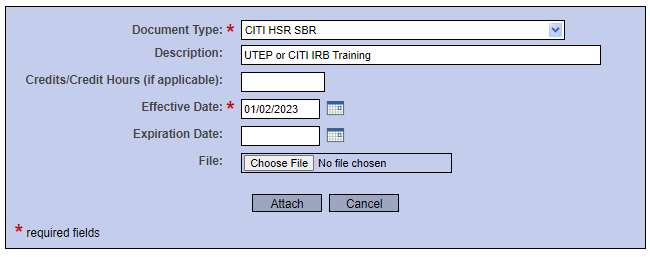
- Please select “CITI HSR SBR” and upload your training certificate. Enter “UTEP or CITI IRB Training” in the description field.
- Enter the effective date then click "Attach".
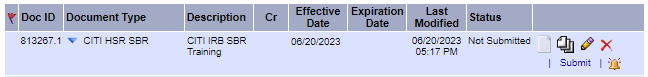
- Once attached, the screen will refresh and Status column will indicate Not Submitted.
- On the lower right hand corner (under the paper icon), click “Submit”.
Responsible Conduct of Research (RCR) modules are for federally supported studies. RCR modules do not meet IRB ethics training requirements.
 You will not receive approval to begin your research project until the HROC office has verified your CITI training.
You will not receive approval to begin your research project until the HROC office has verified your CITI training.NIH Required Training
Good Clinical Practice (GCP) Training
Required for all NIH-funded investigators and staff who are involved in the:
conduct,
oversight,
or management of clinical trials
A clinical trial is defined by NIH as:
A research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes.
All investigators and personnel directly involved in new and ongoing clinical studies that involve the testing of drugs or devices, including all FDA-registered studies as well as investigator-initiated protocols must complete this training.
GCP does not meet IRB training requirements (CITI) for human subjects protection.
Recipients of the training are expected to retain documentation of their training. It is strongly recommended that PIs retain GCP training records for their clinical trial staff.
If you need further assistance or information, please contact the Human Research Oversight and Compliance (HROC) office via e-mail at IRB.ORSP@utep.edu
