After Submission

Here are the most common things that happen after submitting your project/package in IRBNet.
Types of IRB Review
Review Time
10 - 14 days*
*Timeframe varies depending on the completeness or complexity of the project.Only the IRB Chair and/or designee need to review the project. The project must fall into 1 of the 8 categories as defined by 45 CFR 46 and not be higher than minimal risk. Exempt studies are valid for a two-year period. Changes to the project must be submitted to the IRB prior to implementation.
What's Next?
1
Add Missing Documents or Additional Information
The HROC Office will review your project/package for completeness and email you through IRBNet if something is missing or information is required. You will usually have a week to update your project or package.
2
Project/Package Review
The appropriate level of IRB review is primarily based off of the amount of risk to Human Subjects.
- Full Committee Review
- Expedited Review
- Exempt Review
- Limited Review
- Administrative Review
- Facilitated Review
- Ceded Review
3
Project/Package Determinations
May include:
- Acknowledged
- Approved
- Approved with Conditions
- Closed
- Exempt
- Information Required
- Modifications Required
- Not Approved
- Not Human Subject Research
- Referred to Full Board
Projects must have full IRB review to be rejected or not approved.
IRB Determination Expiration
2 Years
All Exempt Review and most Expedited Review
1 Year
Some Expedited Review and all Full Committee Review
Amendments, Reports, Continuations & Closures
UTEP Student/Classroom Research Projects
IRBNet Training Energizer for Researchers
Post Submission Advanced Topics
![]()
Amendment/
Modification
Common Documents Submitted
Amendment Request Form
IRB Project Team Form (Addition/Deletion)
CITI Training
Any previously submitted documents affected by amendment/modification
Project Status
(Continuing Review/Progress Report)Common Documents Submitted
Project Status Report
Research Protocol Application
Informed Consent Form
CITI Training
Protocol
Deviation/
Violation
Common Documents Submitted
Project Status Report
CITI Training
Adverse Event
Common Documents Submitted
Project Status Report
CITI Training
Unanticipated
Problem
Common Documents Submitted
Project Status Report
CITI Training
Closure/Final Report
Common Documents Submitted
Closure Termination Form
1
Create a new package
Click on the title of your project in "My Projects". Then click on "Create a New Package".
2
Go to Designer
Attach all study documents. Please use the current versions available for download in Designer.
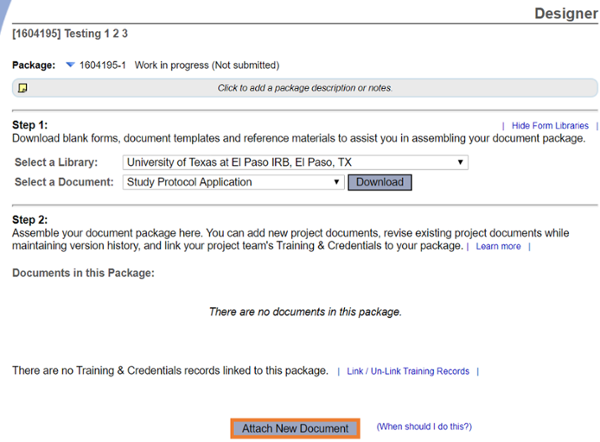
3
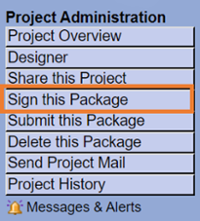
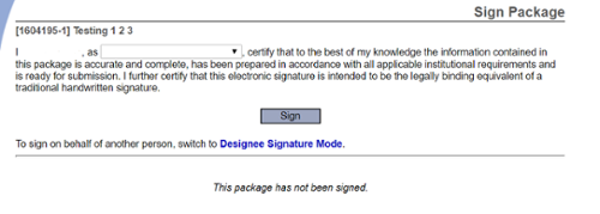
Sign this Package
Sign the package, be sure to choose the role that best describes your role in the research project, e.g. Principal Investigator, Research Coordinator, etc.
4
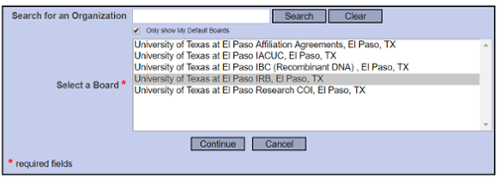
Submit this Package
Select “University of Texas at El Paso IRB, El Paso, TX” and continue to submit.