
MinerAlert

MinerAlert
All Documents may be found on our forms page
Common Documents Submitted
As Needed Documents Submitted
All Documents may be found on our forms page
Common Documents Submitted
All Documents may be found on our forms page
Common Documents Submitted
All Documents may be found on our forms page
Common Documents Submitted
All Documents may be found on our forms page
Common Documents Submitted
All Documents may be found on our forms page
Common Documents Submitted
All Documents may be found on our forms page
Common Documents Submitted
All Documents may be found on our forms page
Common Documents Submitted
Create a new project
Insert the title of your project. Please ensure the title is consistent with the protocol application, consent documents, etc.


Go to Designer
Attach all study documents. Please use the current versions available for download in Designer.


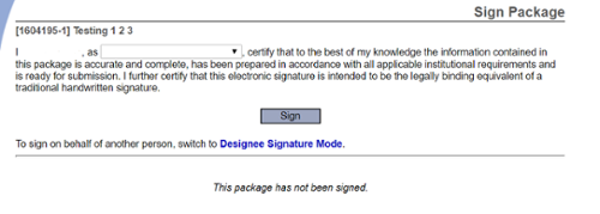
Sign this Package
Sign the package, be sure to choose the role that best describes your role in the research project, e.g. Principal Investigator, Research Coordinator, etc.


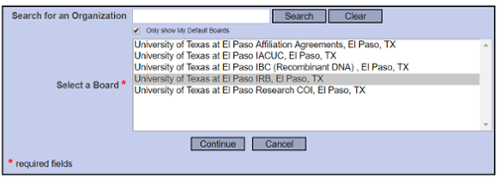
Submit this Package
Select “University of Texas at El Paso IRB, El Paso, TX” and continue to submit.


![]() This next step is mandatory for students.
This next step is mandatory for students.
Optional, but recommended, for Faculty/Staff.
Share this Project.
Select Share.
Students must share their project with their sponsor/faculty advisor.


Share this Project.
Select “University of Texas at El Paso, El Paso, TX” as your organization.
Search for your faculty advisor by their last name.



Share this Project.
Email Faculty Advisor.
Send a gentle reminder to your sponsor/faculty advisor to review and electronically sign your IRB project submission.


Create a new package
Click on the title of your project in "My Projects". Then click on "Create a New Package".

Go to Designer
Attach all study documents. Please use the current versions available for download in Designer.


Sign this Package
Sign the package, be sure to choose the role that best describes your role in the research project, e.g. Principal Investigator, Research Coordinator, etc.

Submit this Package
Select “University of Texas at El Paso IRB, El Paso, TX” and continue to submit.

The IRB Office will review your project/package for completeness and email you through IRBNet if something is missing or information is required. You will usually have a week to update your project or package.
The appropriate level of IRB review is primarily based off of the amount of risk to Human Subjects.
May include:
Timeframe: 10 - 14 Days*
*Timeframe varies depending on the completeness or complexity of the project.Only the IRB Chair and/or designee need to review the project. The project must fall into 1 of the 8 categories as defined by 45 CFR 46 and not be higher than minimal risk. Exempt studies are valid for a two-year period. Changes to the project must be submitted to the IRB prior to implementation.
Timeframe: 2 - 8 Weeks*
*Timeframe varies depending on the completeness or complexity of the project.The expedited category allows selected IRB members to review your project and submit their comments, requirements, and votes individually if it falls into one of the nine federally defined categories. It does not require the IRB to convene a meeting to discuss and vote. Projects are typically granted a two-year approval. A renewal request must be submitted or closed upon completion.
Timeframe: 4 - 12 Weeks*
*Timeframe varies depending on the completeness or complexity of the project.Projects assigned to this review category require the IRB to convene a meeting to discuss and vote on the project for approval. Meetings are held on an as needed basis, typically the last week of the month. Submissions requiring full board review should be submitted to the IRB office no later than the posted deadline. Refer to the posted calendar for tentative dates.
All Exempt and Expedited Review
Full Committee Review